Last week drugnews reported on a warning issued by stryker orthopedics for its lfit v40 femoral head used on several models of its hip replacement implants.
Stryker hip replacement lawsuit canada.
If you have received a stryker hip implant please call 1 888 502 7460 or email strykerclassaction kmlaw ca.
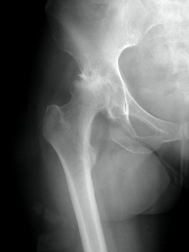
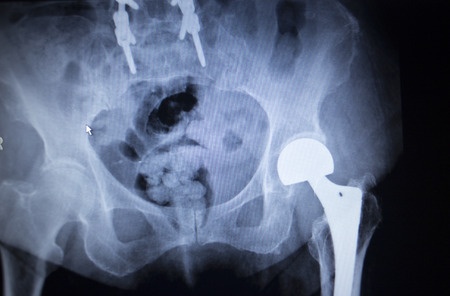
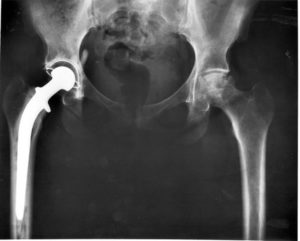
According to the fda medical device recall database the company received multiple complaints from patients who were injured after the taper lock on the femur head failed.
We offer market leading hip replacement implants for total hip arthroplasty including our primary and revision portfolios designed to offer you a wide variety of implants instrumentation and muscle sparing surgical approach options.
As of august 2019 there were nearly 2 000 stryker hip replacement lawsuits in state and federal courts.
In august 2016 stryker issued a voluntary recall on more than 42 500 of its stryker lfit v40 femoral heads the part of the hip implant that attaches to the thigh bone.
This class proceeding concerns allegations that stryker corporation and related companies were negligent in the research design manufacture regulatory licensing sale and post market monitoring of metal on metal hip implants including the rejuvenate and abg ii devices.
The class action was certified by the ontario superior court in toronto on december 8 2015 in ritlop v.
Stryker canada et al.
Stryker s official recall announcement dated july 6 2012 stated that the company decided to recall the hip replacements when it received the disturbing data.
Although the warning was sent only to doctors experts believe the safety risks could result in a.
The company did so over concerns that the parts could fret or corrode resulting in pain swelling and inflammation in the surrounding tissue.
Health canada has issued a recall of components used in total hip replacement surgeries.
Staff writers 6 comments.
The company paid 2 billion in 2014 to settle rejuvenate and abg ii hip lawsuits.
Stryker settles hip implant lawsuits for 1 4 billion in 2012 stryker recalled two metal hip implants the rejuvenate modular and the abg ii modular neck hip stems.
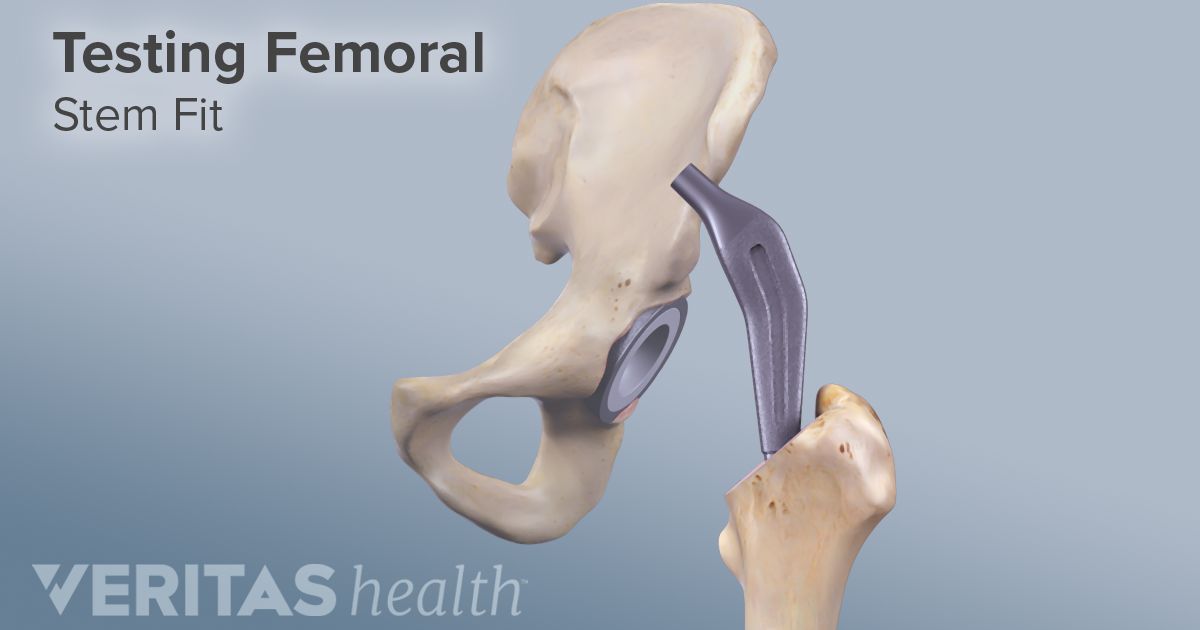
The medical product affected by this recall are stryker lfit v40 femoral heads manufactured before 2011.
Stryker agreed to a confidential settlement of lawsuits over its lfit v40 femoral head in 2018.
A class action lawsuit was started in canada about the stryker rejuvenate modular hip system alleging it was defective and that it failed prematurely.
Canada s fda issues recall of stryker hip implants.
The lfit v40 femoral head is a component used in total hip replacement surgeries.
Hip replacement class action suits in canada target depuy zimmer stryker november 29 2010 written by.