The company notified surgeons and health care facilities that the implants had a higher than expected rate of revision surgery due to a problem known as taper lock failure.
Stryker hip lawsuit 2016.
The 2016 recall affected 42 500 devices.
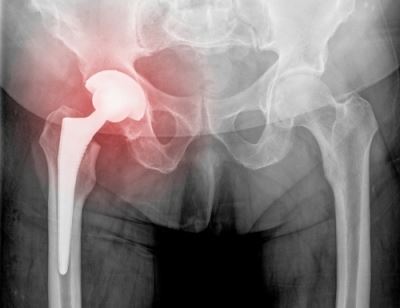
In august of 2016 stryker a manufacturer of various orthopedic medical products including hip prostheses implants used during hip replacement surgery voluntarily recalled their stryker lfit anatomic cocr v40 femoral head components.
Severe pain loss of mobility joint.
Stryker expanded the list in may 2018.
Stryker agreed to a confidential settlement of lawsuits over its lfit v40 femoral head in 2018.
Within a multicounty legislation.
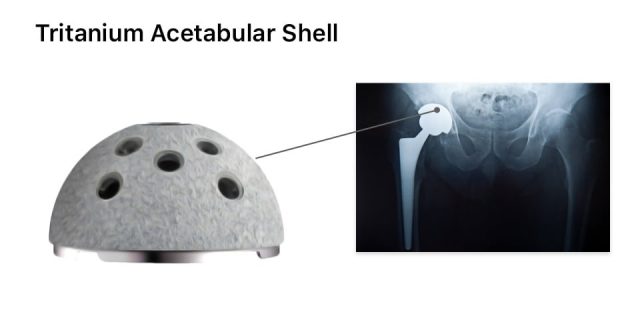
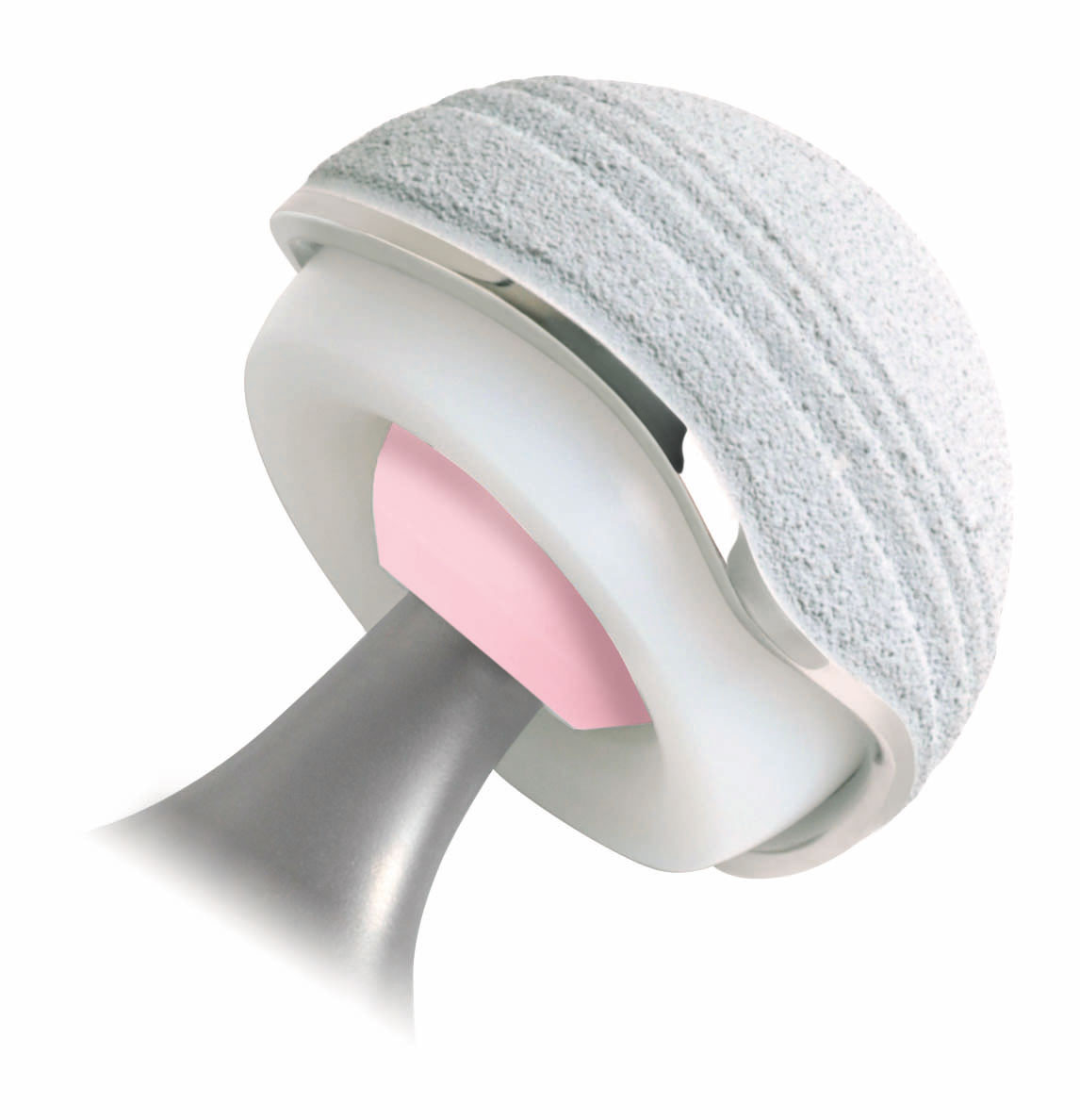
The medical device products involved are the lfit anatomic cobalt chromium cocr v40 femoral heads.
9 2016 according to the fda.
State based stryker lawsuits consolidated in new jersey the new jersey supreme court recently approved consolidation of all pending and future state based hip replacement implant failure lawsuits brought against stryker corp.
The fda issued a class ii recall of the lfit anatomic cocr v40 femoral heads on aug.
Patients also experienced complications of the device including.
Stryker s products have continued to pose dangers to orthopaedic patients.
Stryker hip replacement v40 femoral head recall.
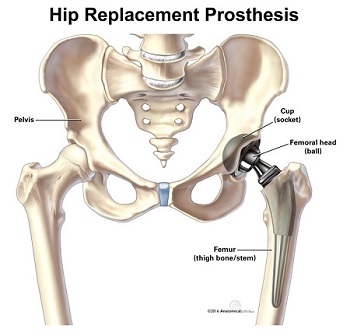
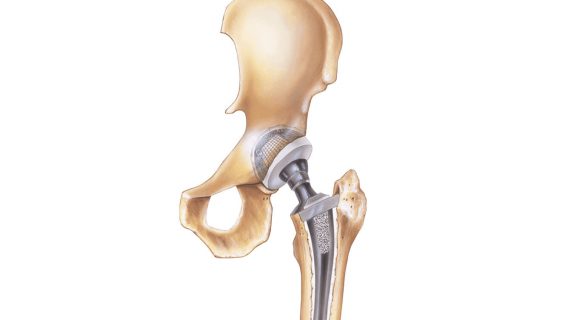
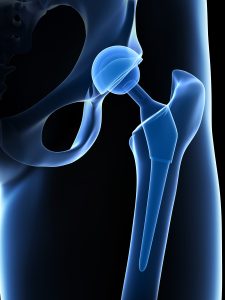
In 2016 stryker issued a recall for their v40 femoral head products which are the metal ball portions of hip replacements that work with hip stems like the accolade tmzf and accolade 2 to replicate the movement of hip joints.
In august of 2016 stryker corp recalled more than 42 000 of its lfit v40 metal femoral head hip implant components.
Stryker hip prosthesis component recall lfit v40 femoral heads.
As of august 2019 there were nearly 2 000 stryker hip replacement lawsuits in state and federal courts.
It added eight additional sizes.