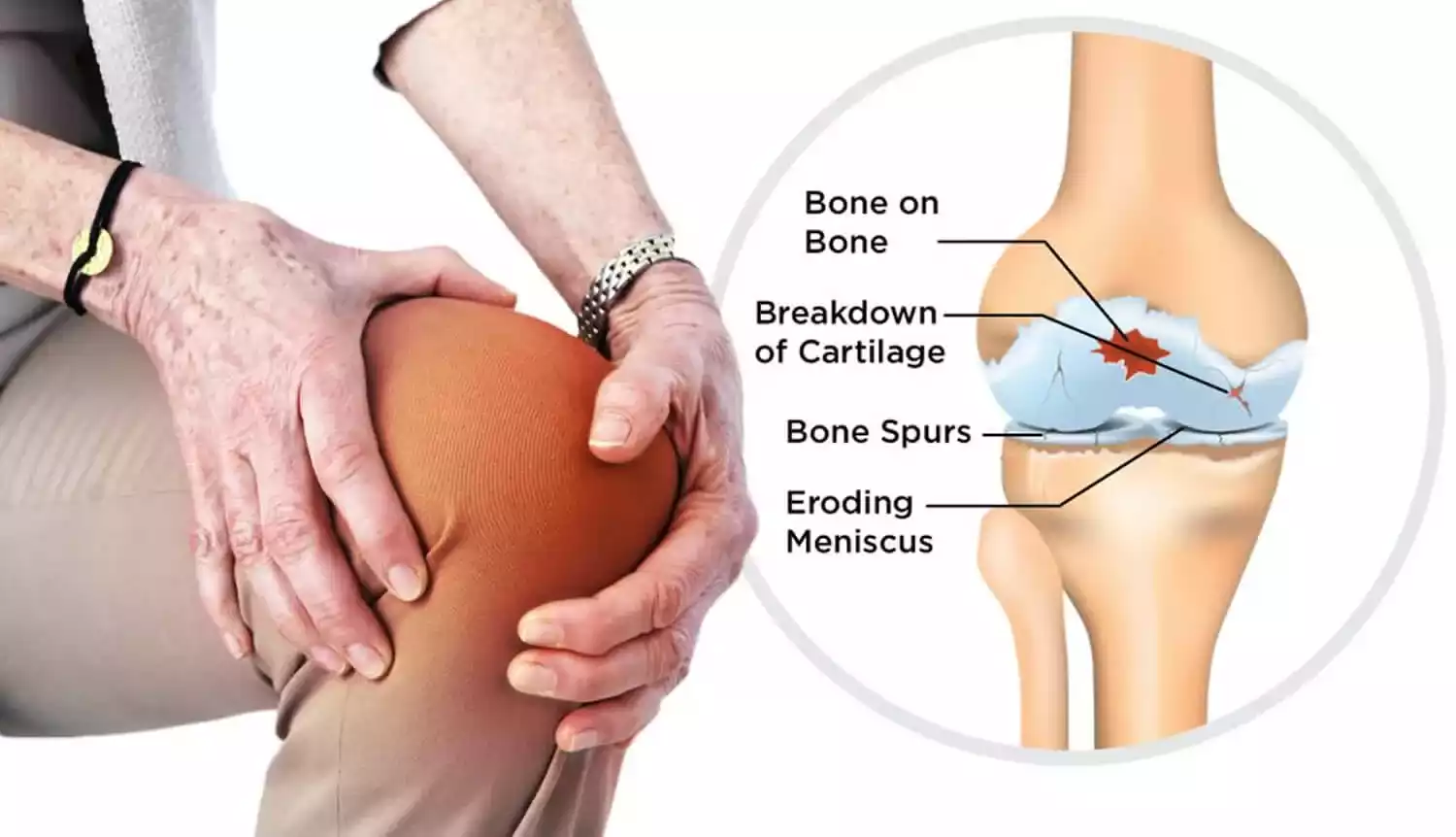
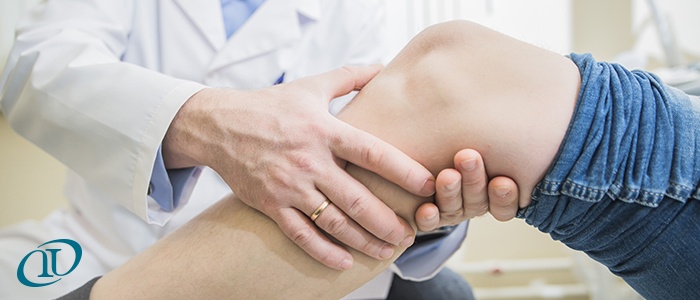
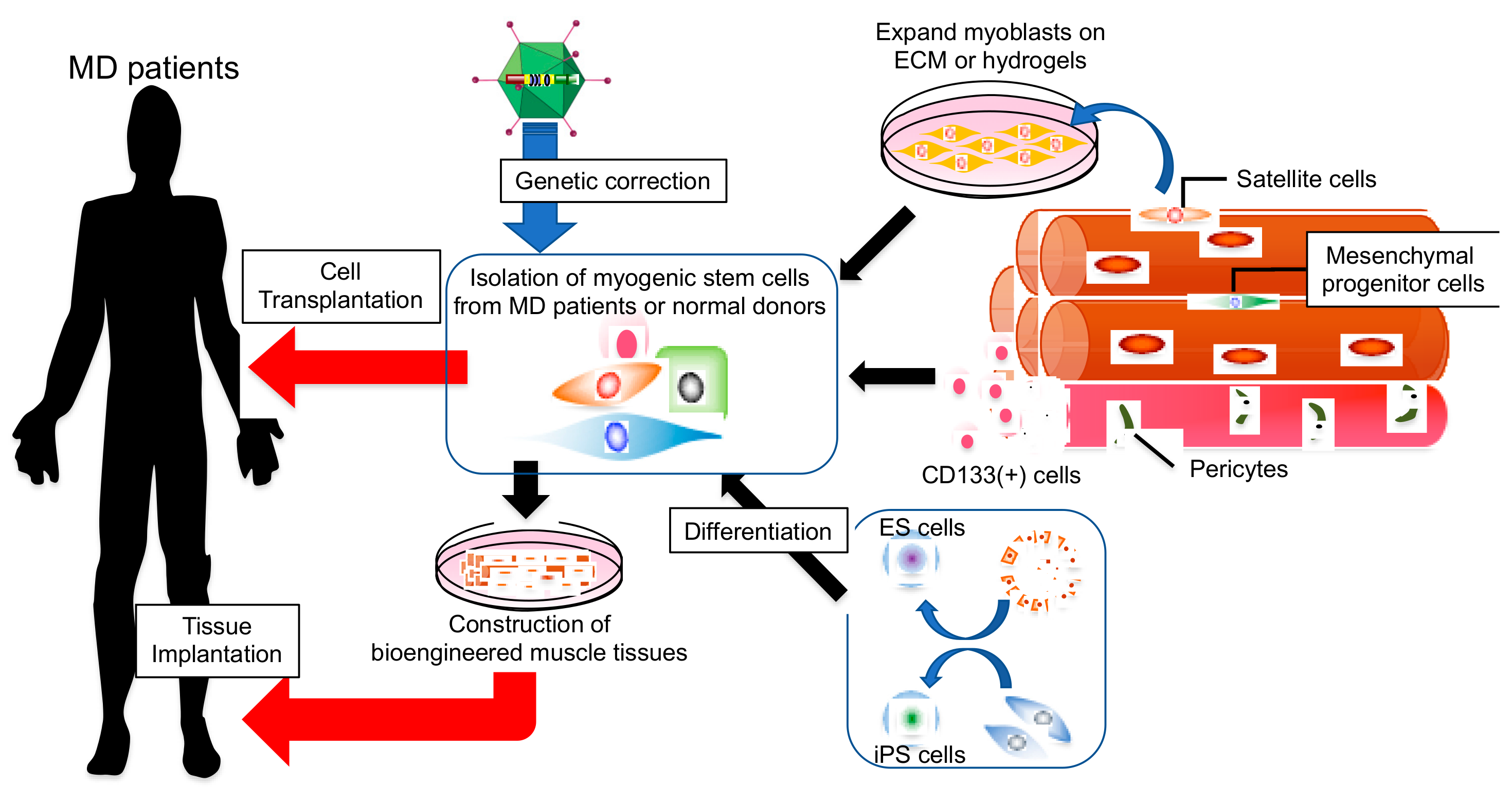
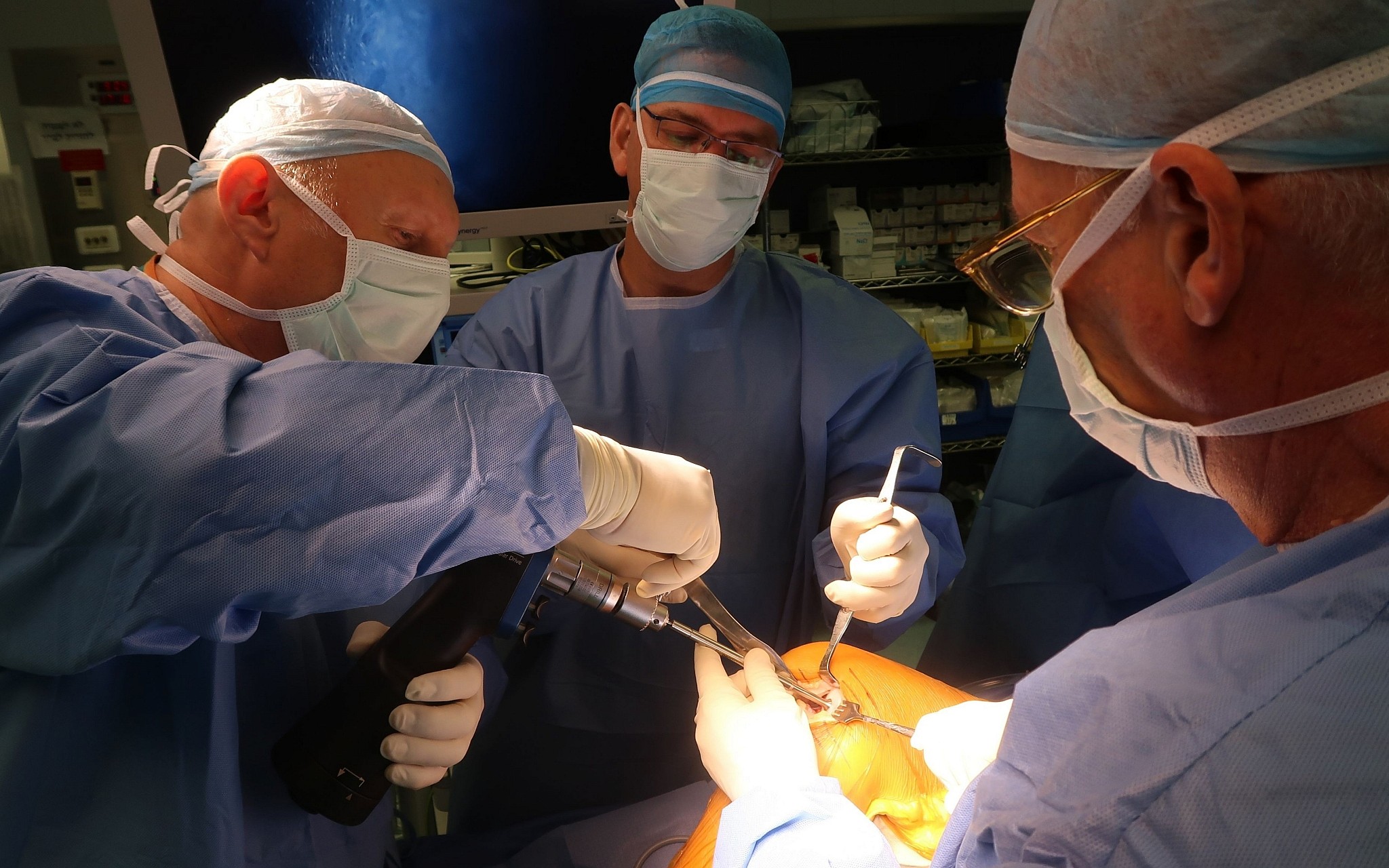
Stem cells collected from the patient s own bone marrow holds great interest as a potential therapy for osteoarthritis of the knee koa because of their ability to regenerate the damaged cartilage.
Stem cell knee cartilage clinical trials uk.
The surgical procedure was performed in two stages ref.
One from 2014 showed that stem cell injections given after surgery to remove torn knee cartilage showed evidence of cartilage regeneration and lessened pain.
San francisco scientists stem cell research could help in covid 19 fight dr.
A groundbreaking clinical trial is now underway at stanford medical center targeting people with injured knees using the patients own stem cells to regenerate cartilage.
Research and clinical trialssee how mayo clinic research and clinical trials.
Longaker and his team at stanford believe they ve isolated a technique that could potentially rejuvenate knee.
Clin cases miner bone merab.
Some research has shown promising results and it may one day become an accepted treatment option.
This cartilage is cultured for four to six weeks so that the cells are increased 30 times in number.
Nam y rim ya lee j ju jh.
Depres tremblay g chevrier a snow m hurtig mb rodeo s.
These cells are then transplanted back into the.
Effectiveness and utility of hyaluronic acid in osteoarthritis.
An fda approved clinical trial.
Current therapeutic strategies for stem cell based cartilage regeneration.
Carried by a virus and delivered by injection into the knee joint of patients with moderate osteoarthritis of the knee.
The first being arthroscopy telescopic examination of the knee to take a small piece of normal cartilage which is cell cultured.
The food and drug administration approved the use of this technique known as recycled cartilage auto allo implantation reclaim in a trial utilizing the stem cell bank in the mayo clinic center for regenerative medicine.
Research into stem cell therapy for treatment of osteoarthritic knee pain is ongoing.
The study is a multicenter trial conducted to compare the effectiveness of corticosteroid control to mesenchymal stem cell preparations from autologous bone marrow concentrate bmac adipose derived stem cells in the form of stromal vascular fraction svf and third party human mesenchymal stem cells manufactured from umbilical cord tissue uct for the treatment of unilateral knee.
Migliore a procopio s.